thumbnail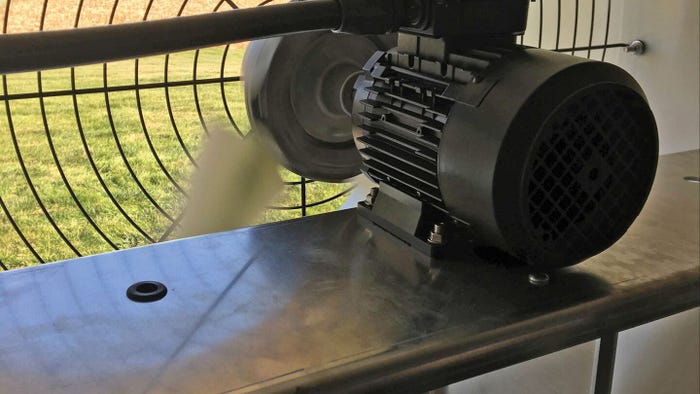
Livestock Management
Protect piglet health during warm weather transitionProtect piglet health during warm weather transition
Get fans, shutters and other cooling system components ready for hotter temperatures.
Subscribe to Our Newsletters
National Hog Farmer is the source for hog production, management and market news

















.jpg?width=300&auto=webp&quality=80&disable=upscale)




















