Influenza virus, a highly variable RNA virus, causes respiratory disease in pigs and other species, including humans. Importantly, in pigs, influenza virus may reduce average daily gain, lead to poorer feed efficiency, increase susceptibility to other infections and represent a constant nightmare for starting pigs off right.
April 30, 2012
Although summer is getting closer, this is no time to lower your guard when it comes to conducting influenza diagnostics to safeguard your herd.
Influenza virus, a highly variable RNA virus, causes respiratory disease in pigs and other species, including humans. Importantly, in pigs, influenza virus may reduce average daily gain, lead to poorer feed efficiency, increase susceptibility to other infections and represent a constant nightmare for starting pigs off right.
Although influenza infections may appear more severe in the fall and winter months, by no means does the virus disappear in the spring and summer. Influenza remains in pig populations throughout the year. Young piglets may be responsible for not only maintaining the virus in the breeding herd, but also for spreading it to distant locations via transport at weaning. In turn, influenza viruses remain endemic in growing pig populations, representing a risk to other pigs, other species and people.
The need to monitor and conduct surveillance in pigs is greater than ever. New strains and new subtypes are often diagnosed, which emphasizes the need to update vaccines and modify control strategies. Fortunately, oral fluids can help make this task easier if the purpose of the testing is to find out whether influenza is present or not, and if so, how long the virus might be present in the population.
Influenza Virus Infections
Oral fluids have proven to be a reliable sample for detecting influenza virus by reverse transcription-polymerase chain reaction (RT-PCR). When compared to nasal swabs, oral fluids can detect low prevalence levels of infection at a relatively low cost.
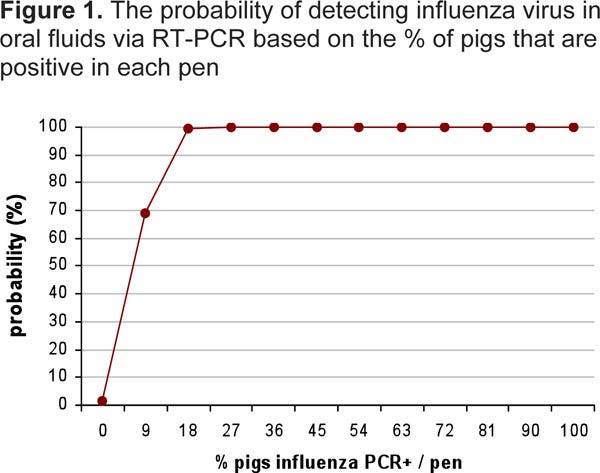
For example, Figure 1 shows a 70% predicted probability of detecting influenza virus with oral fluid sampling in a pen with one positive pig (prevalence of 10%). When the percentage of positive pigs/pen increases to 20%, this probability increases to 99.7%. Overall, there is a high probability to detect influenza virus infection even at low prevalence levels.
In low-prevalence scenarios, a pen oral fluid sample may be negative, while individual pigs in the pen are positive, based on individual animal testing (nasal swabs). So, although individual nasal swabs have higher pen sensitivity than oral fluids, rarely will there be the case when all pigs in a population or pen will be individually sampled due to labor and cost restraints.
Typically, with individual animal testing, a specific sample size is calculated and collected that gives a certain level of confidence in virus detection based on an assumed prevalence. For example, 30 individual samples may be collected in order to detect at least one influenza-virus-positive animal with 95% confidence, assuming a prevalence of at least 10%. Situations in which prevalence is low (few animals infected in a population at a specific point in time) can be observed with influenza virus, especially when one considers the effect of immunity on the ability to detect and isolate the virus.
In particular, pigs with partial immunity induced by either vaccination or natural infection will have lower prevalence infections and therefore the ability to detect the virus will be more difficult. This is important when testing young piglets with elevated maternal immunity and also in vaccinated growing pig populations.
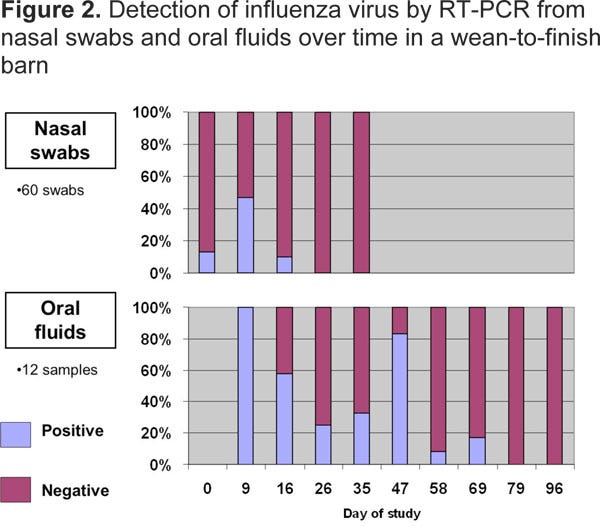
Figure 2 illustrates results from repeated oral fluid and nasal swab samplings from a wean-to-finish group over time. Note that when about 50% of nasal swab samples were positive at Day 9, all oral fluid samples were positive. At Day 16, about 10% of nasal swab samples were positive, while more than half of the oral fluid samples were positive. Interestingly, at Days 26 and 35, influenza virus was not detected via nasal swabs, while oral fluids still identified the group as positive for influenza virus until Day 69.
Additional Points to Consider
Although influenza virus can be isolated from oral fluids, the overall isolation rate remains low compared to that of samples from nasal swabs and respiratory tissue. As a whole, less than 50% of PCR-positive oral fluids will yield viable virus. Therefore, oral fluids should not be seen as a “one shop serves all” when it comes to influenza diagnostics. Nasal swabs and tissue samples from acutely infected pigs are still the preferred sampling techniques for virus isolation, virus typing and virus sequencing.
You May Also Like



